Medical Bits – Vol. 2.4: Your Health and Medical News
You probably don’t need more information about the roaring Pandemic. Over the past several newsletters and updates, we discussed the outbreak of the beta-coronavirus infection, COVID-19 (Coronavirus Infection 2019). Our last update was just 10 days ago, but so much continues to evolve, that we feel obligated to share yet another update. As the Economist outlines, “the interconnectedness of the modern world has been a boon for COVID-19”. Just 12 weeks ago, it took its first steps into a human host in Wuhan and has now caused disease and wrecked economies throughout all continents. “But the interconnectedness may be its downfall, as scientists around the world focus their attention to its RNA genome and the 27 proteins it commands.”
What do we currently know? Timeline of COVID-19:
Source: From Nature.com
- 21 December, 2019, a few patients became ill with severe pneumonia in Wuhan, Hubei province of China.
- 31 December, 2019, a new virus is identified in samples from 4 patients with pneumonia of unknown cause. All patients had been present at the Huanan Seafood Market in Wuhan. Viral genome isolated and sequenced. Initially named 2019-NCoV (Novel Coronavirus 2019).
- 21 January, 2020 – First US case confirmed in Washington State. 30 yo man returning from Wuhan, China.
- 23 January, 2020 – China closes Wuhan.
- 24 January, 2020 – Second US case. 60 yo woman returning to Chicago after visiting China.
- 28 January, 2020 – Human-to-human transmission confirmed in Germany.
- 30 January, 2020 – Human to human transmission confirmed in IL, US.
- 3 February, 2020 – Study of live virus published.
- 14 February, 2020 – Chinese authorities reveal number of infections in medical staff: 1,716 health workers had contracted the virus, 6 of whom died.
- 17 February, 2020 – First case in Africa.
- 25 February, 2020 – U.S. emergency funding for Coronavirus response.
- 26 February, 2020 – Brazil reports first case in South America.
- 28 February, 2020 – Coronavirus spreads to sub-Saharan Africa.
- 4 March, 2020 – Multiple drugs under investigation for Coronavirus.
- 5 March, 2020 – World Bank pledges US$12 billion for Coronavirus response.
- 5 March, 2020 – China study suggests children are as likely to be infected as adults, but most do not become ill.
- 6 March, 2020 – Congress approves US$8.3 billion for coronavirus response to CDC, NIH, FDA.
- 11 March, 2020 – Transgenic animals for CV research in high demand.
- 11 March, 2020 – Coronavirus becomes a pandemic, says WHO.
- 13 March, 2020 – US President declares “national emergency”. It is no longer a “Chinese virus that will blow over by the spring”.
- 17 March, 2020 – First vaccine clinical trials begin in U.S. (National Institute of Allergy and Infectious Diseases (NIAID) and Moderna (biotechnology company in Cambridge, MA) – “launched in record speed,” 66 days from genetic sequencing of virus to the first human injection of the vaccine candidate.
- March 18th, 2020 – Deaths from COVID-19 in Italy surpass those in China.
- March 19, 2020 – No new cases confirmed in Hubei province, China.
- March 31, 2020 – Over 800,000 cases worldwide. Over 80,000 deaths worldwide. For an excellent Pandemic tracker: https://coronavirus.jhu.edu/map.html
Definitions
- COVID-19 refers to the disease, (positive 2019 coronavirus laboratory test regardless of disease signs or symptoms).
- 2019-nCOV was the initial name of the virus, where nCOV stands for novel coronavirus. However, this name is not consistent with virus naming conventions.
- SARS-CoV-2 is the Gen-bank name for the virus, (96% identical in nucleotide sequence to SARS-CoV, the cause of SARS in 2002-3).
Transmission
- Person-to-person spread occurs via respiratory droplets, similar to influenza. According to a WHO – China report, the rate of symptomatic infection after confirmed exposure ranges from 1-5%.
- The mean incubation period is 5 days but may take up to 14 days after exposure for symptoms to appear.
- Most infections are not severe. In an earlier report from the Chinese Center for Disease Control and Prevention that included 44,500 infections, 81% were mild, 14% were severe with multi-lobar pneumonitis and 5% had critical disease developing respiratory failure.
- Initial case-fatality rate was 2.3% but likely much lower, as many cases are asymptomatic which lowers the case-fatality rate but allows for the creation of a larger reservoir, as asymptomatic individuals may transmit the disease and help propel the epidemic.
- Case fatality rate has ranged from almost 10% in Italy to 0.1%in Germany. There are multiple factors to consider. Age of population, access to technology, public health organization, etc.
- More than 80% of cases are mild and only 3% of all clinical cases occur in people under 20.
- Cruise ship outbreak off the coast of Japan, all the passengers and staff were tested, 17% of the population tested positive, but 50%were asymptomatic at the time of diagnosis.
- On Feb 28th, 2020, Li et al. reported the initial cohort of patients, describing that the median age was 59 years, with higher severity of illness in the elderly and among those with chronic conditions. There were no cases reported in children below 15 years of age, likely due to mild disease which escaped detection and implies that the case-fatality rate is a lot lower than initially perceived as discussed above.
- Another article by Guan et al. reported on Feb 28th, 2020, a mortality of 1.4% among 1100 patients with laboratory-confirmed disease but the case definition required pneumonia.
Mortality
- Out of almost 50,000 cases reported and analyzed:
- 15% mortality in patients over 80.
- 8% mortality in patients over 70.
- 4% mortality in patients over 60.
- 1% mortality in patients over 50.
- 0.4% mortality in patients over 40.
- Less than 0.2% mortality in patients younger than 40.
- 5% overall mortality in patients with underlying respiratory disease or malignancy.
Testing and Diagnosis
Poor initial national response and dismal leadership from the White House. For a comprehensive discussion and review of our National poorly coordinated Pandemic response: https://www.nytimes.com/2020/03/28/us/testing-coronavirus-pandemic.html?campaign_id=34&emc=edit_sc_20200331&instance_id=17208&nl=science-times®i_id=73874292&segment_id=23391&te=1&user_id=7c61cdd369e5e89648c819ddce494fef
- CDC initially inflexible on testing guidelines (needed travel or exposure history):
- Their test required slow, overnight RT-PCR reaction with a specific model, designed poor primers and became aware a month later.
- FDA had stringent rules on testing:
- Approved only CDC test; refused working tests from WHO and other countries
- Required CDC to retest results of other labs
- Allowed academic labs to develop own tests on 2/29/20
- Approved rapid Roche test on 3/13/20
- Individuals who develop high fever and associated progressive breathlessness warrant testing.
- Individuals without high fever or breathing difficulty do not require immediate testing except if important for epidemiologic reasons or to limit infection to vulnerable groups.
- If you or a loved one develops respiratory symptoms, obviously COVID-19, influenza, another respiratory virus, or pneumonia are possibilities and sometimes impossible to tell them apart unless testing is completed.
Clinical Presentation
- All patients with clinical disease, presented with fever, frequently above 102 F / 39 C.
- 60% reported a dry cough.
- 35% reported body aches.
- 30% reported difficulty breathing, usually after 5 days of acute illness.
- The white cell count can be elevated or low. It may cause significant and transient decline in lymphocyte counts below 1500.
- Some patients have elevated liver function test.
- Remember that minimal symptoms or even no symptoms are possible.
Isolation
What if you have traveled or a family member has developed an acute respiratory illness? Do they have COVID-19. Do we have to keep distance in order to avoid becoming vectors and spreading the disease?
- Test-based – may discontinue isolation;
- Spontaneous resolution of fever and improvement in cough and shortness of breath and
- Negative COVID-19 testing twice > 24 hours apart.
- Non-Test based – may discontinue isolation:
- 7 days have passed since symptoms first appeared and
- 3 days have passed since recovery of symptoms.
Goals of Mitigation in Pandemics
- Approximately 5% of total infected (not diagnosed cases) may require hospitalization.
- 2.5% may require support in Intensive Care Units.
- Average hospital stay is 2 weeks (1 week after diagnosis).
- Calculations suggest biggest infection surge will occur weeks of late May 2020 and early June 2020.
- Goal: slow down doubling time from 1 week to >8 weeks, such that at peak < 500,000 hospitalizations per week.
- Most important is to handle the current Pandemic and prevent the next Pandemic – Ban Wet Markets and be prepared.
- Abbott has developed a rapid serologic test for the detection of IgM and IgG against SARS-Co-2 which requires only a finger prick or a drop of blood and has high sensitivity and specificity, providing results within 15 minutes. We have requested this diagnostic kit but will likely not be available for at least another 1-2 months. This test may help determine who already had the disease and give us a better sense of the disease “denominator.”
Treatment
Mostly limited to supportive care measures at present:
- Mostly limited to supportive care measures at present:
- Intravenous hydration.
- Symptomatic relief.
- Care of complications – Oxygen supplementation.
- Mechanical ventilator support in patients with progressive pneumonitis.
Clinical Trials and Novel Treatments
- There are over 105 clinical trials underway in China and as of today, March 31, 2020 there are 194 clinical trials listed on www.clinicaltrials.gov to evaluate several new, re-purposed medications and vaccination trials.
- RNA Vaccine – Phase I clinical trial launched March 10th at Kaiser Permanente Washington Health Research Institute in Seattle.
- Remdesivir, promising nucleotide analogue with activity against COVID-19. Previously used against SARS, but epidemic controlled and medication “shelved”. Now used for moderate to severe infection. Clinical trials ongoing.
- Favipiravir is an RNA protease inhibitor which has in-vitro activity and has been shown to accelerate viral clearance (median time decreased from 11 to 4 days) and accelerated radiographic improvement on chest radiography in an open-label, non-randomized study.
- Lopinavir-ritonavir has in-vitro activity, but a study out of China in 200 patients with severe pulmonary disease, failed to demonstrate clinical benefit or mortality. NO role at this point.
- Other agents:
- Chloroquine/hydroxychloroquine, in vitro activity but no proven benefit at this point.
- Camostat mesylate blocks viral proteases
- Tocilizumab – Actemra – Interleukin-6 pathway inhibitor.
- Toremifene
- Irbesartan – Losartan
- Melatonin
- Paroxetine
- Mesalamine
- Eplerenone
- Carvedilol
- Sirolimus
- Umifenovir
- Home management and observation may be appropriate for the majority of patients who are likely to develop relatively mild disease.
- As summarized in an Editorial article on the New England Journal of Medicine on February 28th, 2020, the information available suggests that COVID-19 is similar to a severe seasonal influenza or a pandemic influenza, which has a case fatality rate of around 0.1% and up to 0.7% (1 and up to 7 out of 1000 individuals may perish as a result of the disease or more frequently complications). One problem is the inaccurate overall denominator as we do not fully know how many patients are or have been infected. Mortality can be as high as 10% as documented in Italy.
- This is a lot lower than the mortality documented from the other coronaviruses emerging in the 21st century, SARS – 9-10% or MERS – 35%. We should count our blessings and be grateful. Hard to imagine but, it could have been a lot worse.
- 5% of patients will develop severe disease and up 2.5-5% of total patients may need Intensive Care Unit Support.
Vaccine Candidates
Excellent review: https://www.nejm.org/doi/full/10.1056/NEJMp2005630?query=featured_coronavirus
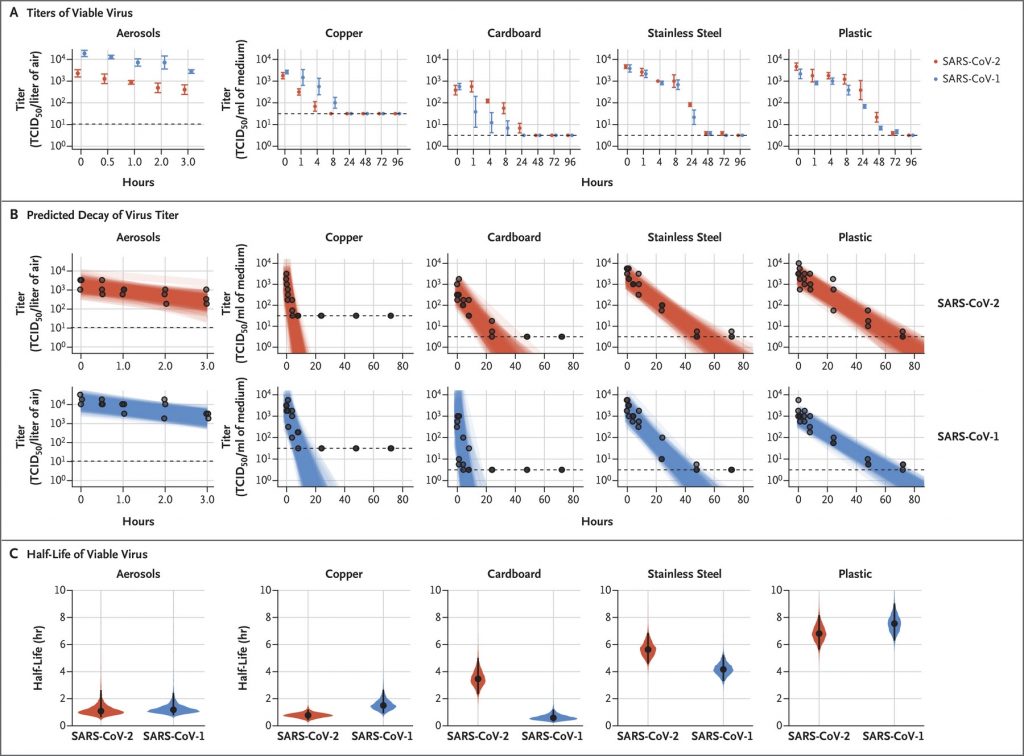
Viability of SARS-CoV-2 on different surfaces
https://www.nejm.org/doi/full/10.1056/NEJMc2004973?query=featured_home

What should our patients do?
WHO and CDC recommend
- Use standard contact and droplet precautions with mouth and face protection.
- What should our patients with existing respiratory conditions and vulnerabilities be alert to? The usual symptoms of influenza – fever.
- –runny nose – dry cough and in particular, increasing trouble breathing, which may indicate progression of disease and development of inflammatory viral pneumonitis (damage of the pulmonary alveolar-capillary membrane where gas exchange takes place).
- If you have these symptoms, stay home. Treat your symptoms with over the counter remedies and remain well hydrated. Apply Grandma’s treatment! If you develop increasing breathlessness, intractable cough and associated high fevers over 102-103, grab a mask to cover your face and proceed to your Emergency Department for evaluation.
- Do not take ibuprofen or NSAIDS, as they may aggravate the severity of illness. Best to use acetaminophen or paracetamol to control fever.
Additional Resources
The International Coalition for Epidemic Preparedness and Innovations (CEPI) is already working on up to eight vaccine candidates. One has launched as indicated above. If you would like to keep abreast of this rapidly moving field, see the references at the bottom of this summary.
COVID-19-NJEM
Debunking Myths: Q&A
Myth: Wearing a respiratory mask at all times will keep me safe!!!
Not true! Those ill with an acute respiratory infection should wear masks to decrease the aerosolization of viral particles, but not for those who are healthy, as regular surgical masks do not prevent the penetration of viruses. They may limit droplets and prevent entrance but it is unlikely that you will keep a mask on for the duration of your commute or your journey and it is likely you will touch it and remove it contaminating your hands and from there, your mucosal surfaces. The N-95 masks which are likely to catch viral particles and most suspended droplets are effective, but serious business to wear for longer than a few minutes and must be fitted, placed properly and importantly, removed accurately to prevent contamination.In one study completed in a Japanese hospital, the use of surgical-type masks by health care workers did not reduced the frequency of colds or respiratory illnesses and subjects using masks were more likely to complain of headaches.
Myth: I cannot re-use my N-95 mask!
Not true. Keep it clean / don’t bend it and clean the front with antiseptic wipes.
Myth: If I get COVID-19, I will develop permanent lung damage!
NOT true. As indicated above, most patients have low grade disease. Patients who develop severe pneumonitis may have modest lung damage that improves over time.
Myth: I can get COVID-19 not only once but several times and severity increases!
NOT true! Once you have the disease, your body mounts an effective immunogenic response and you will not suffer it again!
Myth: There are no diagnostic tests to help me determine if I had the disease!
As indicated above, we now have a way to identified immunoglobulins triggered by infection with COVID-19 and able to determine who has become immunized against the disease through a rapid IgG / IgM serologic test performed in a few drops of blood.
Myth: Taking an antihypertensive such as Lisinopril, enalapril, losartan, irbesartan or olmesartan, may increase my risk of COVID-19
Since the virus uses ACE2 receptors to attach itself to the respiratory mucosae! But on the other hand, increasing ACE2 receptor expression, common in patients with hypertension may reduce the risk of severe pulmonary infection. This is an active area of investigation and at present the recommendation is to continue with your usual treatment. In fact, there are two clinical trials looking at the use of Losartan and Irbesartan to decrease the severity of illness.
In the meantime, keep cool, do not panic, eat a nutritious and diverse diet, stay active and be happy! And try to make the best lemonade with the “lemons” nature has thrown our way! COVID-19 is already here and is infecting members of our community. But panic is not a rational response and is not likely to help. This too shall pass! Remember that the only certainty in life is… death… and the only fountain of youth proven by science, experience and millennia are exercise, laughter, humor and a good positive attitude!
Enjoy every minute of this most interesting JOURNEY and cherish this family time!!! And there is one good thing that COVID-19 has brought to the Picone Tribe!
Let me introduce…COVY!

Cheers!
Carlos Picone, M.D.
5215 Loughboro Rd, Suite 400
Washington, DC 20016
email: cpicone@chevychasepulmonary.com
As long as you live, keep learning how to live.
References
- https://www.nejm.org/coronavirus
- https://www.cdc.gov/coronavirus/2019-nCoV/index.html
- https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/
- https://www.nature.com/articles/d41586-020-00154-w]
PPT – Covid19-Science and Treatment. Drs. Steven Gordon and Serpil Erzurum – Cleveland Clinic.
We are now offering Telemedicine appointments and happy to arrange a “remote” consultation /visit and see you at the office if required.